So what if Hydrogen isn’t a noble gas? It might not open the door for them female gases or pick up the bill at restaurants for that matter. But we’ll have you know that it is the most abundant (75%!) element in the universe. It makes stars burn (that’s hydrogen there burning at the centre of our sun). We wouldn’t have sunlight on our planet if it wasn’t for Hydrogen. And everybody would be racking up ridiculous electricity bills.
Source: Wikimedia Commons
Water is essential for any life to exist on this planet. And everybody knows that water is two-part hydrogen, and one-part oxygen.

Source: Wikimedia Commons
Give it to humans to tarnish the good name of Hydrogen by fusing it to do this.

Source: http://4.bp.blogspot.com/-K06GBzXrVrc/Th84GP26FQI/AAAAAAAAC8c/Dpxvi5jNcCo/s1600/aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa.jpg
Let’s take a look at the hydrogen atom. In school, we’ve all learned it looks something like this! Well, prepare to get your mind blown because it’s all wrong!
Ramchandani J / SOL
Electrons don’t lie in a neat little orbit like a planet orbiting the sun.

Wikimedia Commons
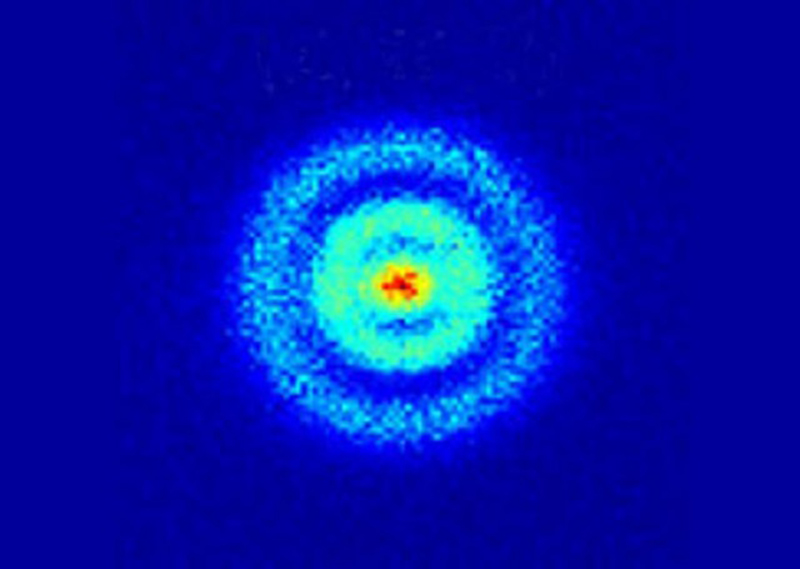
They lie in a cloud of probability. This is a real cloud of probability of hydrogen’s ONE electron (first time it was ever snapped in May 2013 using a quantum microscope!)
Something called the uncertainty principle makes it REALLY difficult for researchers to make a picture of an atom. To get this picture, researchers had to take thousands of snapshots of the hydrogen atom. The blue dots are the less probable positions of finding the electron and the the red dots are the most probable positions. Totally different from our text books right?
But wait, then where are the neutrons and protons? Well, that’s even more complicated. If the atom were the size of a football field, the nucleus (which have the neutrons and protons) is just a pin head at the centre.

Wikimedia Commons
And what occupies the rest of the atom?
Well… nothing really. It’s mostly just space. That’s right. Watch this to really understand!
Think About It
We’re all made out of atoms. Atoms are 99% empty space. Atoms come together to form molecules and molecules come together to form compounds. And the entire visible material world we see is made of one compound or another. So does that mean that we are 99% empty space too? And when I am touching someone, am I really touching them?
Learn More
An Atom Undressed: New Scientist Article | You Can’t Touch Anything | What is touch?
About the Authors
Jaya Ramchandani works on several astronomy and physics outreach projects. She is also a co-founder of Sirius Interactive, a language solutions company for researchers.
Jonathan Dias freelances for magazines and papers like Maxim and the Herald, writing about women, food and gadgets. He is also attempting to write a cook book that involves a lot of bacon and beer.